Warning of counterfeit Ozempic® - Update 31.10.2023
Update 31/10/2023
The manufacturer of Ozempic®, Novo Nordisk A/S, has published a comprehensive patient information on the identification of original Ozempic® and Saxenda® pens on 30/10/2023.
The Federal Office for Safety in Health Care is not aware of any incidents with Saxenda® in Austria at the moment. However, the same recommendations as listed below apply. Patients should report any suspicions or indications of possible counterfeit products to the BASG enforcement department (enforcement@basg.gv.at).
Links
Manufacturer's press release to identify original Ozempic® and Saxenda® pens of 30/10/2023: Presse & Medien (novonordisk.at) and Presseaussendung_Counterfeit_Novo Nordisk.pdf
Safety information (DHPC) of 02/11/2023: https://www.basg.gv.at/fileadmin/redakteure/06_Gesundheitsberufe/DHPC/2023/231102_Ozempic_Saxenda_F%C3%A4lschung.pdf
Update 23/10/2023
The existing BASG warning dated 12/10/2023 and warning of counterfeit Ozempic® - Update 19.10.2023 is supplemented by the occurrence of Ozempic® counterfeits in several patients in Austria and reminds that the only legal source is on prescription and via public pharmacies and physicians in charge of in-house pharmacies.
The prescription-only medicinal product Ozempic® contains the active ingredient semaglutide and is approved for the treatment of patients with type 2 diabetes. Recently, Ozempic® has been increasingly used as a "weight loss" medication, for which the medicinal product is not approved. This has led to a limited availability of Ozempic® for diabetic patients. This shortage is apparently being exploited by criminal organizations to place counterfeits of Ozempic® on the market. Counterfeit medicines can be hazardous to health. Due to the untested quality of the counterfeit drug, possible impurities and unknown ingredients, these counterfeits can also be life-threatening.
The BASG informs that there are already Ozempic® counterfeits that have reached patients in Austria. The BASG has received initial reports that several patients had to be treated in hospital after using suspected counterfeit Ozempic®. The reported serious side effect with hypoglycemia and seizure is an indication that the product falsely contained insulin instead of the active ingredient semaglutide.
Together with the Criminal Intelligence Service Austria, the BASG is therefore issuing an urgent warning against counterfeit "weight loss" medication from dubious sources of supply. According to the current state of investigation of the Criminal Intelligence Service Austria, the affected batch was obtained by the person from a physician based in Austria. In this case, the pre-filled pens probably came from a source other than a pharmacy. According to the current state of investigation, stocks of the affected batch may still be in circulation or may have been obtained by other physicians via this illegal channel. Patients who have obtained Ozempic® pre-filled pens directly from physicians not in charge of in-house pharmacies should contact them immediately.
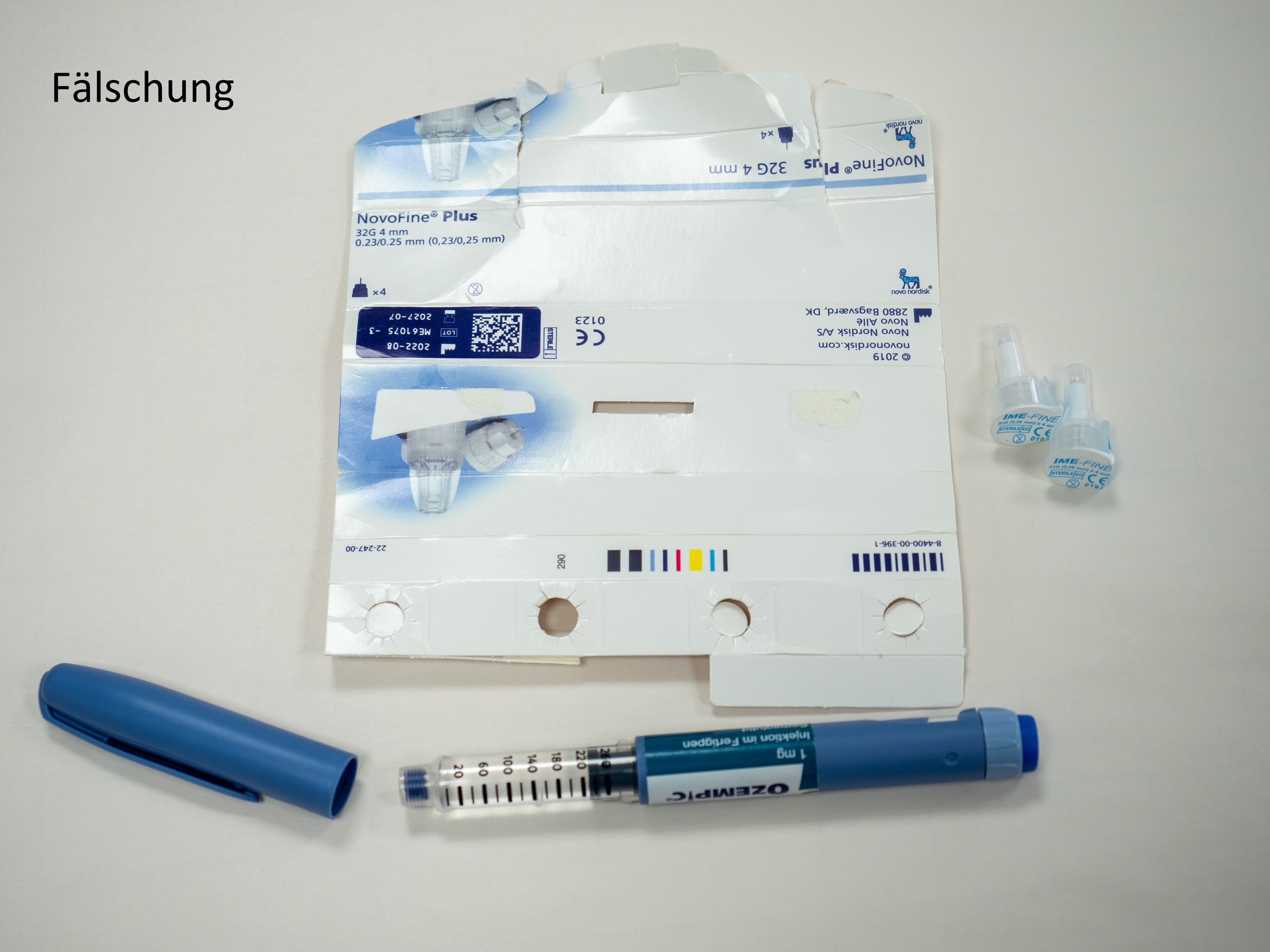
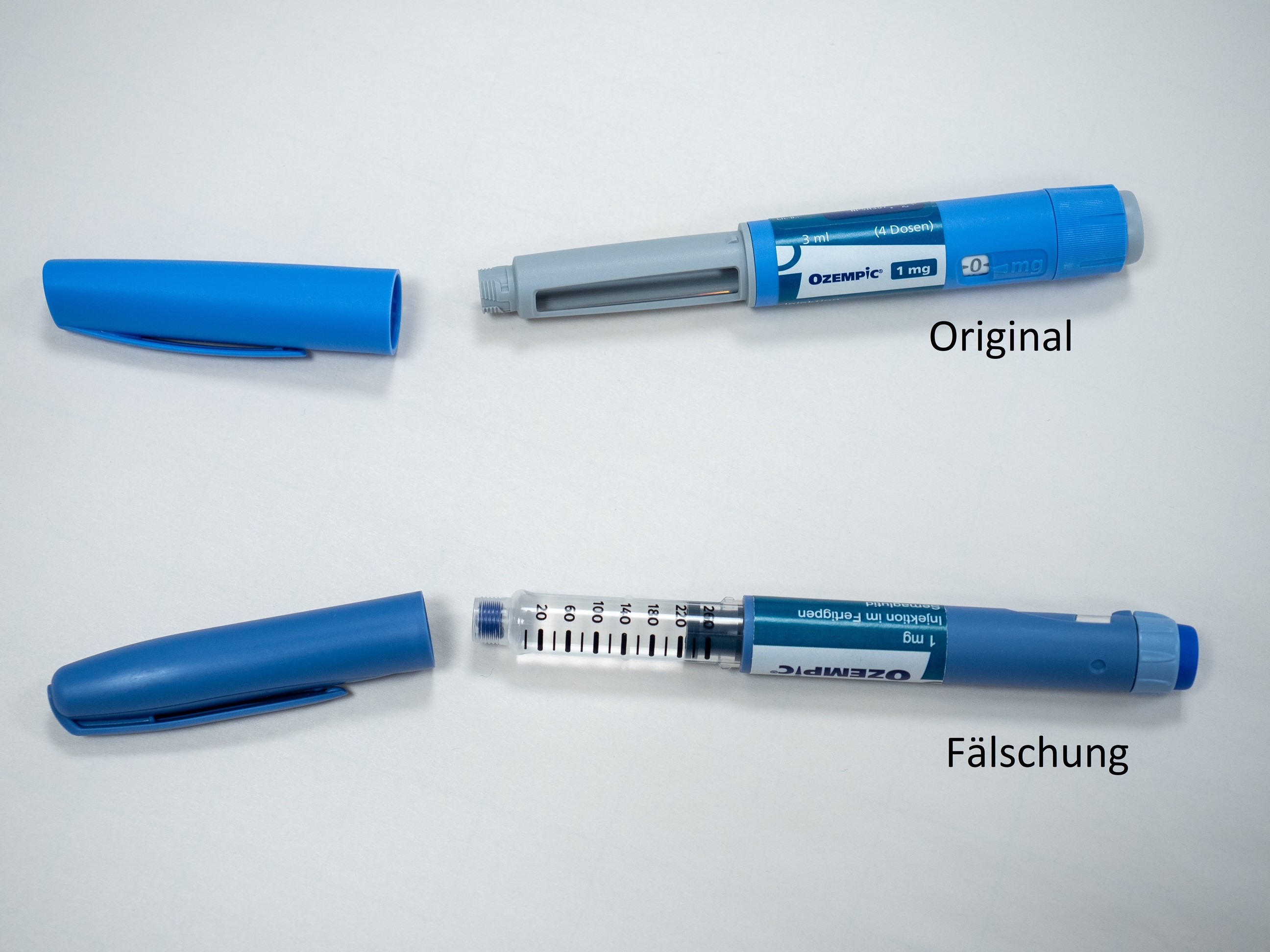
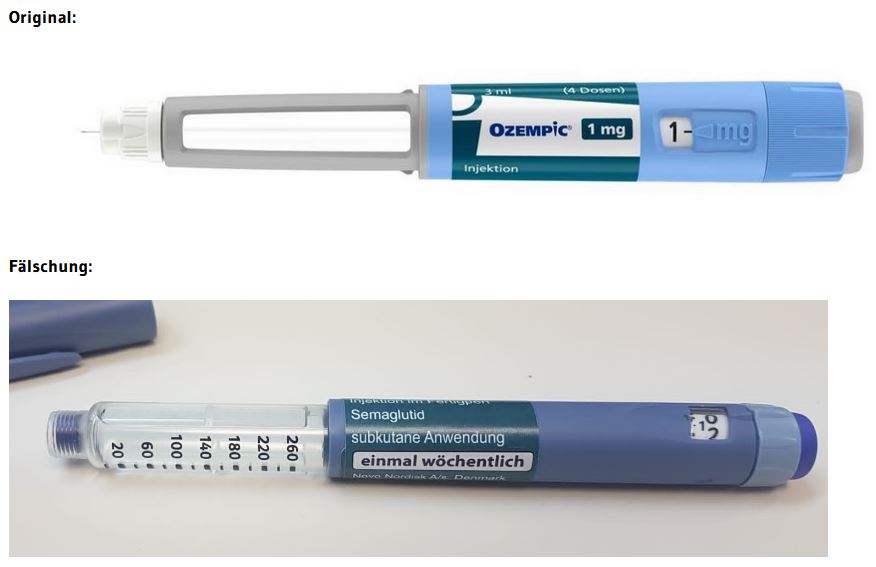
According to current knowledge, the suspected counterfeits are Ozempic® packs of strength 1 mg (Ozempic 1 mg injection solution in a pre-filled pen). At present, it cannot be ruled out that other packs with a different strength are affected. Counterfeit Ozempic® pre-filled pens are easy to distinguish from the real Ozempic® (see photos at the end of this article).
Possible distinguishing features according to the warning of the Criminal Intelligence Service Austria
- The counterfeit pen differs in colour from the original, the "blue" is darker than the original.
- The safety window is different. In the counterfeit it is completely transparent, in the original it is covered with grey paint.
- The dose adjustment ring is different. It can be extended in the counterfeit, which is not possible in the original.
- The enclosed needles both have a length of 4 mm, the labelling of the original needle is 32G and not 31G.
The BASG assumes that there are also counterfeits of Ozempic® that look different or are not offered as a pre-filled pen. On the outer packaging, the counterfeit is difficult or impossible to detect. It is therefore important to emphasise that counterfeits in circulation do not necessarily have to have only these pictured optical characteristics, but could possibly also have a different appearance. Information regarding medicines not obtained through pharmacies can be reported at Bundeskriminalamt@bmi.gv.at.
Recommendations of the BASG
Recommendations for healthcare professionals
- Physicians not in charge of in-house pharmacies who have dispensed Ozempic® to patients should use the photos provided to check whether they are possible counterfeits and check the source of supply. However, it cannot be ruled out that there are other counterfeits in circulation that look different. It should be noted that physicians are authorised to dispense medicines to patients if they are medical samples or a physician in charge of in-house pharmacies. Physicians and physicians in charge of in-house pharmacies may only obtain their medicinal products from a public pharmacy in the European Economic Area (§ 31 para. 3 ApothekenG and § 57 para. 3 ÄrzteG).
- Pharmacists and physicians in charge of in-house pharmacies are advised that before dispensing Ozempic® to patients, the secondary packaging must always be checked for authenticity using the electronic security feature as part of the serialization and verification of medicinal products. Any suspected counterfeiting of a medicinal product must be reported immediately to the BASG. Further cases of Ozempic® counterfeiting cannot be ruled out. There are no indications that an affected product has been dispensed to patients by legal pharmacies. However, pharmacists should provide patients with additional information on how to recognize counterfeit packages (e.g. based on the package leaflet and published images).
- Health professionals should also remind their patients to buy medicines only from legal pharmacies.
The BASG points out that potential side effects and/or health damages that may occur as a result of the use of counterfeit Ozempic - not only, but also - may presumably be related to the insulin that may be falsely contained therein. When treating potential clinical cases, it must therefore be taken into account therapeutically that these side effects may also be the result of an overdose with insulin or a resulting hypoglycemia with sometimes serious symptoms.
Recommendations for wholesalers:
- Based on these developments, wholesalers are recommended to check the secondary packaging for authenticity using the electronic security feature as part of the serialization and verification of medicinal products. Any suspicion that a drug is counterfeit must be reported immediately to the BASG. Further cases of Ozempic® counterfeiting cannot be ruled out.
Recommendations for patients:
- Patients who have obtained Ozempic® directly from physicians not in charge of in-house pharmacies are advised to bring back the pack to the physician concerned. The physician should check the authenticity of the medicinal product and, if necessary, take further action.
- Patients are again strongly and urgently warned against any unauthorised purchase of Ozempic® and especially against purchases from dubious sources. Genuine Ozempic® can only be obtained by prescription and dispensing through a public pharmacy or through physicians in charge of in-house pharmacies.
- Since it is not possible to check pre-filled pens from non-legal sources, they should not be used. Counterfeits can be hazardous to health and potentially life-threatening due to their untested quality and potential impurities and unknown ingredients.
- The BASG furhtermore advises all patients that Ozempic® is a prescription-only medicinal product. It is therefore not possible to order Ozempic® on the internet, where only over-the-counter medicines can legally be obtained. Any order placed on the internet for Ozempic® is therefore not only unlawful and illegal, but also carries a very high probability of obtaining a counterfeit Ozempic® product. These counterfeits can be hazardous to health and potentially life-threatening due to untested quality and potential contaminants and unknown ingredients. Legal mail-order pharmacies (which by law are only ever authorized to ship over-the-counter medicines) therefore do not ship Ozempic®.
- In contrast to the large number of illegal pharmacies that appear on the internet ("rogue pharmacies"), legal mail-order pharmacies for ordering only over-the-counter medicines can always be recognized by their officially registered and tested mail-order pharmacy logo - and thus distinguished from health-endangering and illegal internet pharmacies.
- Patients are therefore strongly and urgently warned against any unauthorized ordering of Ozempic® on the internet. Genuine Ozempic® can only be purchased by prescription and dispensed by a public pharmacy. This is the only way to ensure that the product purchased is an approved, well-tested, safe and effective and therefore authentic medicine.
- There is no evidence that the counterfeit products were supplied to patients by legal pharmacies.
- Patients are urged not to use Ozempic® pens suspected of being counterfeit. Patients should report any suspicions or indications of possible counterfeit products to the BASG enforcement department (enforcement@basg.gv.at).
The BASG would like to point out that Ozempic® is a prescription-only medicinal product. It is therefore not possible to order it on the internet, where only over-the-counter medicines can legally be obtained. Any order for Ozempic® on the internet is therefore not only unlawful and illegal, but also carries a very high probability of obtaining a counterfeit Ozempic® product. These counterfeits can be hazardous to health and potentially life-threatening due to untested quality and potential impurities and unknown ingredients. Legal mail-order pharmacies (which by law are only ever authorized to ship over-the-counter medicines) therefore do not ship Ozempic®. Legal mail-order pharmacies for ordering exclusively over-the-counter medicines can always be recognised by their officially registered and tested mail-order pharmacy logo, in contrast to the large number of illegal pharmacies appearing on the Internet (so-called "rogue pharmacies") - and can thus be distinguished from health-endangering and dubious internet pharmacies.
If all these safety measures are observed, counterfeit Ozempic® can be prevented from reaching the patient and cases of damage as described above can be avoided.
The European Medicines Agency (EMA) has also issued a warning about counterfeit Ozempic® pens in Europe. BASG continues to work closely with the investigative authorities involved at national and international level.
Update 19/10/2023
The Federal Office for Safety in Health Care (BASG) informs about new developments concerning counterfeits of the medicinal product Ozempic® (original manufacturer: Novo Nordisk A/S). The existing warning of the BASG dated 12/10/2023 is updated due to the occurrence of Ozempic® counterfeits in patients in Austria.
The prescription-only medicinal product Ozempic® contains the active ingredient semaglutide and is approved for the treatment of patients with type 2 diabetes. Recently, Ozempic® has been increasingly used as a "weight loss" medication, for which the medicinal product is not approved. This has led to a limited availability of Ozempic® for diabetic patients. This shortage is apparently being exploited by criminal organizations to place counterfeits of Ozempic® on the market. Counterfeit medicines can be hazardous to health. Due to the untested quality of the counterfeit drug, possible impurities and unknown ingredients, these counterfeits can also be life-threatening.
The BASG informs that there are already Ozempic® counterfeits that have reached patients in Austria. The BASG has received a first report that a patient had to be treated in hospital after using suspected counterfeit Ozempic®. The reported serious side effect with hypoglycemia and seizure is an indication that the product falsely contained insulin instead of the active ingredient semaglutide.
According to current knowledge, the suspected counterfeits are Ozempic® packs of strength 1 mg (Ozempic 1 mg solution for injection in pre-filled pen). At present, it cannot be ruled out that other packs with a different strength are affected. Counterfeit Ozempic® pre-filled pens can be easily distinguished from the genuine Ozempic®. BASG assumes that there are also counterfeits of Ozempic® that look different or are not offered as a pre-filled pen. It is difficult or impossible to detect the counterfeit on the outer packaging. Therefore, it is important to emphasize that counterfeits in circulation do not necessarily have to have exclusively these optical characteristics, but could possibly have a different appearance in some cases.
Recommendations of the BASG
Recommendations for healthcare professionals
- Pharmacists and physicians in charge of in-house pharmacies are advised that before dispensing Ozempic® to patients, the secondary packaging must always be checked for authenticity using the electronic security feature as part of the serialization and verification of medicinal products. Any suspected counterfeiting of a medicinal product must be reported immediately to the BASG. Further cases of Ozempic® counterfeiting cannot be ruled out. There are no indications that an affected product has been dispensed to patients by legal pharmacies. However, pharmacists should provide patients with additional information on how to recognize counterfeit packages (e.g. based on the package leaflet and published images).
- Health professionals should also remind their patients to buy medicines only from legal pharmacies.
The BASG points out that potential side effects and/or health damages that may occur as a result of the use of counterfeit Ozempic - not only, but also - may presumably be related to the insulin that may be falsely contained therein. When treating potential clinical cases, it must therefore be taken into account therapeutically that these side effects may also be the result of an overdose with insulin or a resulting hypoglycemia with sometimes serious symptoms.
Recommendations for wholesalers:
- Based on these developments, wholesalers are recommended to check the secondary packaging for authenticity using the electronic security feature as part of the serialization and verification of medicinal products. Any suspicion that a drug is counterfeit must be reported immediately to the BASG. Further cases of Ozempic® counterfeiting cannot be ruled out.
Recommendations for patients:
- The BASG advises all patients that Ozempic® is a prescription-only medicinal product. It is therefore not possible to order Ozempic® on the internet, where only over-the-counter medicines can legally be obtained. Any order placed on the internet for Ozempic® is therefore not only unlawful and illegal, but also carries a very high probability of obtaining a counterfeit Ozempic® product. These counterfeits can be hazardous to health and potentially life-threatening due to untested quality and potential contaminants and unknown ingredients. Legal mail-order pharmacies (which by law are only ever authorized to ship over-the-counter medicines) therefore do not ship Ozempic®.
- In contrast to the large number of illegal pharmacies that appear on the internet ("rogue pharmacies"), legal mail-order pharmacies for ordering only over-the-counter medicines can always be recognized by their officially registered and tested mail-order pharmacy logo - and thus distinguished from health-endangering and illegal internet pharmacies.
- Patients are therefore strongly and urgently warned against any unauthorized ordering of Ozempic® on the internet. Genuine Ozempic® can only be purchased by prescription and dispensed by a public pharmacy. This is the only way to ensure that the product purchased is an approved, well-tested, safe and effective and therefore authentic medicine.
- There is no evidence that the counterfeit products were supplied to patients by legal pharmacies.
- Patients are urged not to use Ozempic® pens suspected of being counterfeit. Patients should report any suspicions or indications of possible counterfeit products to the BASG enforcement department (enforcement@basg.gv.at).
If all these safety measures are observed, counterfeit Ozempic® can be prevented from reaching the patient and cases of damage as described above can be avoided.
The European Medicines Agency (EMA) has also issued a warning about counterfeit Ozempic® pens in Europe. BASG continues to work closely with the investigative authorities involved at national and international level.
Links
BASG warning of 12/10/2023: https://www.basg.gv.at/marktbeobachtung/amtliche-nachrichten/detail/warnung-vor-gefaelschtem-arzneimittel-ozempic
EMA warning of 18/10/2023: https://www.ema.europa.eu/en/news/ema-alerts-eu-patients-healthcare-professionals-reports-falsified-ozempic-pens
BfArM warning: https://www.bfarm.de/DE/Arzneimittel/Arzneimittelinformationen/Rapid-Alert-System/Arzneimittelfaelschungen/Ozempic/_node.html
Swissmedic warning: https://www.swissmedic.ch/swissmedic/de/medicrime/news/warnings/smc-warnt-vor-faelschungen-von-ozempic-pens.html
Manufacturer's press release to identify original Ozempic® and Saxenda® pens of 30/10/2023: Presse & Medien (novonordisk.at) and Presseaussendung_Counterfeit_Novo Nordisk.pdf
Manufacturer's warning: https://www.novonordisk.at/content/dam/nncorp/at/de/press-and-media/20231026-patient.inneninformation-final.pdf
Safety information (DHPC) of 02/11/2023: https://www.basg.gv.at/fileadmin/redakteure/06_Gesundheitsberufe/DHPC/2023/231102_Ozempic_Saxenda_F%C3%A4lschung.pdf
Enquiries (professional):
Dr. Christoph Baumgärtel, Tel.: +43 505 55-36004
E-Mail: christoph.baumgaertel@ages.at
Enquiries (for media):
Communication Management, Tel.: +43 505 55-25000
E-Mail: presse-basg@basg.gv.at