Batch release
What is batch release?
For certain proprietary medicinal product, Official Control Authority Batch Release (OCABR) must be obtained from the Austrian Official Medicinal Control Laboratory (OMCL) before they can be placed on the market.
For proprietary medicinal products approved in Austria, batch release certificates from other OMCLs within the EU/EEA countries and Switzerland may also be recognised if the approval in the country of release is in accordance with the Austrian product specification.
The following proprietary medicinal products intended for human use are covered by these provisions:
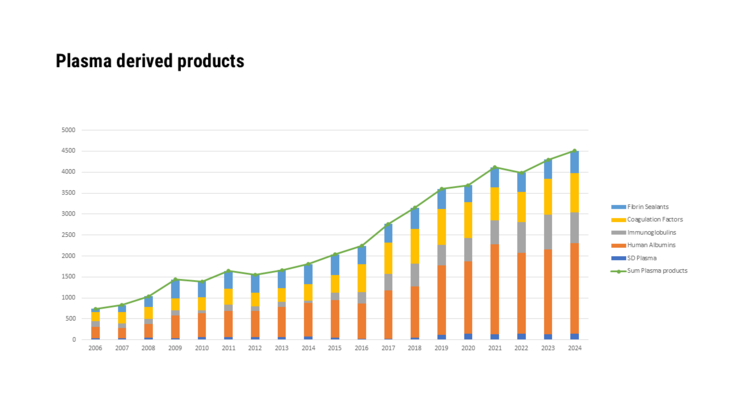
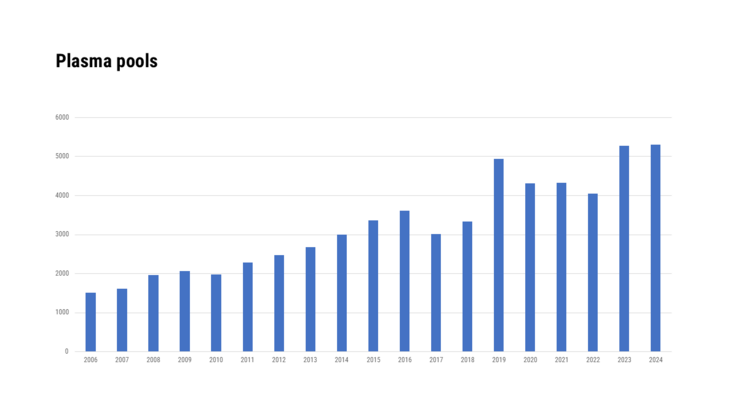
- Medicinal specialities prepared using human blood or blood plasma as a starting material
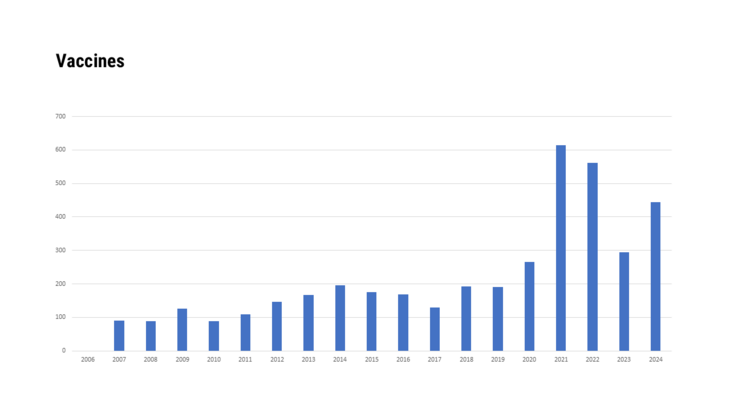
- Immunological medicinal specialities consisting of vaccines, toxins, sera or allergens, in so far as these are
- live vaccines
- medicinal products used for the primary immunisation of young children or other risk groups
- medicinal products used in immunisation programmes in the context of public health; or
- medicinal products which are newly authorised or produced by means of novel techniques or which are new to a particular manufacturer. These proprietary medicinal products shall be subject to batch release for a transitional period to be determined. These medicinal specialities must be approved by the Federal Office for Safety in Health Care by official decision.
- The batch release requirement also applies to medicinal specialities intended for use on or in animals, provided that they are immunological medicinal specialities consisting of vaccines, toxins, sera or allergens and are intended for the prevention of notifiable animal diseases in accordance with the Animal Diseases Act.
Who must apply for batch release?
- Companies with autorisation from the BASG to produce, control, sale and distribute Medicinal Products
- Companies which are approved contract partners with the EWR to produce, control, sale and distribute Medicinal Products
- Importeurs of Medicinal Products (especially for clinial trails)
Which medicinal products are concerned?
Human
§26 of the Austrian Medicinal Products Act requires batch release for the following medicinal products for human use:
- Medicinal products that were produced using human blood or blood plasma as source material, as well as
- immunological medicinal products consisting of vaccines, toxins, sera or allergens, if they are
- life vaccines,
- medicinal products used for primary immunisation of infants or other risk groups,
- medicinal products used within the scope of immunization programs of the public health sector, or
- medicinal products with a new marketing authorisation or produced by a novel technique or a technique that is novel for a specific manufacturer. These medicinal products are subject to batch release for a specified transition period.
Veterinary
Tierarzneimittelgesetz §23.
(1) Immunological medicinal products for veterinary use intended for use on or in animals, consisting of vaccines, toxins, sera, or allergens and intended for the prevention of notifiable animal diseases in accordance with §2 (1) Tiergesundheitsgesetz 2024, BGBl. I Nr. 53/2024, shall be subject to batch release.
(2) §26 AMG shall apply accordingly.
How is the procedure for receipt of a batch release certificate?
If a batch shall be marketed the applicant submits the application form together with the complete protocol and enough sample material for testing at the OMCL.
From September 2022, there will be a new portal through which batch release applications can be submitted. At https://eservices.basg.gv.at, authorized users can submit their applications in a straightforward manner. For more information, please contact chargen@basg.gv.at! For eServices, please visit this link for more information.
Subsequently all required parameters are tested accordingly to the product specific OCABR guidelines at the laboratory. The completeness and validity of the submitted batch protocol will be reviewed by an assessor according to the marketing authorization.
After receipt of the complete application, compliant test results and assessment a batch release certificate is granted.
Forms
Please submit your applications via the portal at https://eservices.basg.gv.at. If you do not find your product, contact chargen@basg.gv.at or, in urgent cases, use one of the following forms for the first submission.
Special Services to Shorten and Simplify Batch Release
Parallel Application
The applicant can first hand in samples and later on the batch protocol when testing is completed. Batch release will usually be performed within one working day.
e-Submission of Application
All documents necessary for batch release can be easily transmitted via the portal or by eMail.
Certificates for Auxiliaries & Excipients
Protocols for excipients can be handed in without samples for testing. Certificate are granted free of charge.
e-Transmission of Release Certificates
Certificates are sent by e-mail or fax before they are sent postal to shorten the time to marketing for the applicants.
Export Certificates
Export certificates are granted for products intended for marketing outside the EU.
Meetings with Applicants
Periodical Meetings concerning process improvements are held upon request.
Legal basis and guidelines for batch release
Available in German from the Austrian Legal Information System (RIS)
- Clinical trial according to the Medicinal Products Act §8
- Notifiable disease according to Austrian Animal Diceases Act §16
- Batch release according to the Medicinal Products Act §26
Available from the European Directorate for the Quality of Medicines & HealthCare (EDQM)