Quality of medicines
Legal basis
Proof of the required quality of a human or veterinary medicinal product is, in addition to its proven efficacy and safety, a fundamental prerequisite for marketing authorisation. Marketing authorisation holders and manufacturers are legally obliged to ensure that the quality of their medicinal products always corresponds to the latest scientific findings, even after marketing authorisation has been granted. This applies equally to all products, including generics.
The required quality documentation essentially comprises the composition, development, manufacture, control, packaging and storage of finished products and active substances, as well as any relevant documentation for medical devices or medical device components that are placed on the market in combination with the medicinal product.
All quality aspects must be mapped in Module 3 of the dossier and must correspond to the structure of the CTD (Common Technical Document), which is specified in the EudraLex Notice to Applicants, Volume 2B.
Furthermore, the content of the European Pharmacopoeia, guidelines, Q&A documents, etc. must be taken into account:
- European Pharmacopoeia (EP, https://pheur.edqm.eu/home), as well as the Austrian Pharmacopoeia (ÖAB, Österreichisches Arzneibuch - BASG)
- Guidelines for human medicinal products
- Guidelines for veterinary medicinal products
- Q&A documents
European and Austrian Pharmacopoeia:
The European Pharmacopoeia defines recognised quality standards for medicinal products and their components (active substances, excipients, packaging materials). The Austrian Pharmacopoeia is a national supplement to the European Pharmacopoeia. Both pharmacopoeias are declared binding in Austria by ordinance in accordance with the Pharmacopoeia Act.
Guidelines:
Quality guidelines reflect a harmonised approach on how to interpret and apply the legal requirements for proof of quality. Applicants and marketing authorisation holders are strongly encouraged to follow these guidelines. Deviations from guidelines must be fully justified.
Q&A documents:
Q&A documents reflect the harmonised position on issues that may be interpreted differently or require clarification. Q&As should be read in conjunction with the European Pharmacopoeia, quality guidelines, etc.
Active pharmaceutical ingredient documentation
Module 3 of the authorisation dossier is divided into two sections that deal separately with information on the active substance and the finished product. While the information on the finished product is always product-specific and is stored in the authorisation dossier itself, the active substance documentation can be provided in three ways:
- Complete documentation: The quality documentation for the active substance is available in full in the authorisation dossier.
ASMF (active substance master file):
The quality documentation for an active substance is often submitted in the form of an ASMF (Active Substance Master File, formerly DMF "Drug Master File"), which divides the information on the manufacture and control of the active substance into two parts. While the Applicant's Part (AP) is made available to the applicant by the active substance manufacturer in order to assume responsibility for the quality of the active substance, the Restricted Part (RP) is only accessible to the authorities. This physical separation serves to keep the intellectual property of the active substance manufacturer confidential. Questions and deficits that frequently arise in connection with the submission and use of ASMFs are summarised in the FAQ below: https://www.basg.gv.at/fuer-unternehmen/zulassung-life-cycle/faq-zulassung-life-cycle/asmf
CEP (certificate of suitability to the monograph of the European Pharmacopoeia):
The active substance manufacturer submits the dossier with all data on active substance manufacture to the European Directorate for the Quality of Medicines (EDQM), where the assessment is carried out centrally. However, this is limited exclusively to active substances for which a monograph exists in the European Pharmacopoeia. Following a positive assessment, the active substance manufacturer is issued with a CEP, which certifies that the quality of the active substance is adequately controlled by the corresponding pharmacopoeia monograph. In addition to purely chemical substances, CEPs can also be issued for herbal active substances (herbal CEPs).
A special case are so-called TSE CEPs, which concern substances with a particular risk of transmitting transmissible spongiform encephalopathies (TSE). These mainly include substances of animal origin, such as gelatine. In this case, the CEP certifies that the substances fulfil the criteria of Ph. Eur. Monograph 1483 "Products with risk of transmitting agents of animal spongiform encephalopathies".
For further information, please refer to the public EDQM document "How to read a CEP" (PA/PH/CEP (15) 31).
Questions and deficits that frequently arise in connection with the submission and use of CEPs are summarised in the FAQ: www.basg.gv.at/fuer-unternehmen/zulassung-life-cycle/faq-zulassung-life-cycle/cep
Validity of the active ingredient and finished product manufacturers
What needs to be considered with regard to the validity of active ingredient and finished product manufacturers after authorisation?
According to the Medicinal Products Act, marketing authorisation holders must ensure the required quality of their medicinal products.
If the active substance documentation is covered by an EDQM certificate (CEP), the continuous validity of this document must be ensured. If CEPs are temporarily invalidated/suspended (status: "suspended") or withdrawn (status: "invalid"), they are no longer authorised as documentation of the required quality of the active substance. Suspension or cancellation of a CEP can be queried on the EDQM website ("Actions on CEPs") or the database ("Certification").
Similarly, the quality of the active substance is no longer guaranteed if "Active Substance Master Files" (ASMFs) are used after GMP inspections with a negative outcome. Corresponding non-compliance reports (NCRs) are published in the Eudra GMDP database.
In these cases, the BASG considers that the quality of the active substances concerned is no longer guaranteed and therefore the provisions of the Medicinal Products Act are no longer complied with.
If a GMP inspection with a negative outcome concerns a finished product manufacturer, a Non-Compliance Report (NCR) is published in the Eudra GMDP database as a result. Here, too, the provisions of the Medicinal Products Act are no longer complied with or, in the opinion of the BASG, the quality of the finished products concerned is no longer guaranteed.
What measures are to be taken in the event of suspension or cancellation of a CEP or a Non Compliance Report (NCR) for an active substance or finished product manufacturer?
In the event of a suspension or cancellation of a CEP or as a result of a GMP inspection with a negative outcome (or issued NCR), the current manufacturers for the active substance or finished product as well as planned further measures must be submitted to the Federal Office for Safety in Health Care (BASG).
If necessary, a different/new active substance manufacturer or a different/new finished product manufacturer including corresponding documentation must be submitted by means of a change application and approved by the BASG.
Enquiry note
If you have any queries regarding NCRs, please contact am-qualitaetsmangel@basg.gv.at. If you have any queries regarding affected products, please contact lcm@basg.gv.at.
Risk assessment of nitrosamines in medicinal products
Information for marketing authorisation holders: Assessment of the risk of possible nitrosamine impurities in medicinal products for human use
Nitrosamines are chemical compounds that are classified as probably carcinogenic to humans based on animal studies. After impurities with nitrosamines were identified in various medicinal products, a scientific evaluation of the Committee for Medicinal Products for Human Use (CHMP) was started in September 2019 in accordance with Article 5 (3) of Regulation (EC) No. 726/2004, which was finalised in July 2020.
As a result, it was determined that the risk of the presence of nitrosamines as impurities in human medicinal products must be reduced as far as possible and must be less than or equal to the applicable limit value.
Furthermore, all marketing authorisation holders of human medicinal products with chemical and biological active substances should evaluate them with regard to the risk of possible contamination with nitrosamines and take any necessary measures.
Further information can be found
- on the homepage of the European Medicines Agency "EMA - Nitrosamine Impurities"
- and the Coordination Group "CMD(h) - Nitrosamine Impurities"
Austria's practical approach to the Article 5(3) referral on nitrosamines
The risk assessment is carried out in three successive stages
- Stage 1 - risk evaluation
- Stage 2 - testing, if necessary
- Stage 3 - Modification of the authorisation, if necessary
It should be noted that marketing authorisation holders are expected to review and reconsider the outcome of the risk evaluation as soon as new information becomes available, even after the responses to step 1 and/or step 2 have been submitted.
In general, the procedure for human medicinal products differs depending on the active substance:
- Chemically synthesised active substances
- Biological active substances
Chemically synthesised active ingredients
Step 1 - Risk evaluation
The result of the risk evaluation regarding possible impurities with nitrosamines in authorised human medicinal specialities with chemically synthesised active substances was to be submitted to the BASG by 31 March 2021 at the latest.
A corresponding procedure was created in the eService for each affected product. If this has not happened in individual cases, please contact nat@basg.gv.at. This also applies if a risk evaluation procedure has already been completed, but an update of the previous notification is required due to new information.
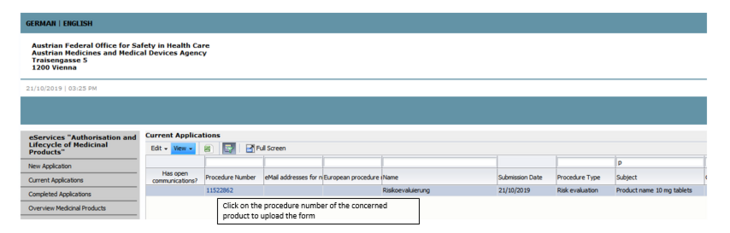
The name of the medicinal product can be found in the subject line of each affected product (see Figure 1).
On the CMDh homepage you will be provided with 2 documents with the respective document designations (Step 1 - no risk identified, Step 1 - risk identified).
Please select the appropriate document for your product, fill it in completely and then upload it using the procedure provided in the eService, specifying the correct document type (Step 1 - no risk identified, Step 1 - risk identified) (see Figures 2 and 3).
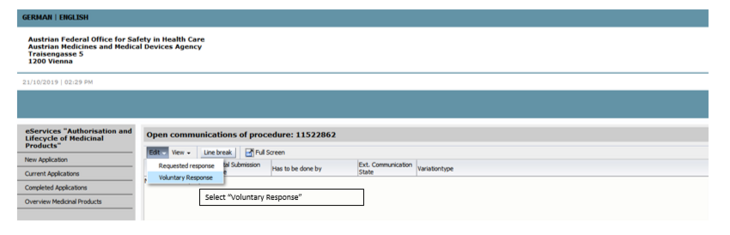
To upload the documents, open the procedure via the respective procedure number and select voluntary subsequent submission.
The Excel table listed in the corresponding practical guidance (see CMDh website) is - as also stated there - not mandatory in Austria at this time and therefore does not necessarily have to be uploaded.
Please use only the procedure described above for your reports in Austria.
If you do not have access to the portal or if you have any questions, please contact
Step 2 - Testing
CAVE: only relevant if a risk was identified in Step 1.
In the eService, a corresponding procedure was created for each affected product in Step 1 and - if a risk has been identified - this procedure remains open.
CMDh will provide you with 2 documents with the respective document designations (Step 2 - no nitrosamines found, Step 2 - nitrosamines found).
Please select the appropriate document for your product, fill it in completely and then upload it to the procedure provided in the eService, specifying the correct document type (Step 2 - no nitrosamines found, Step 2 - nitrosamines found) (see Figures 2 and 3).
To upload the documents, open the procedure via the respective procedure number and select voluntary subsequent submission.
Please use only the procedure described above for your notifications in Austria.
Step 3 - any changes to the proprietary medicinal product
Variations required due to the risk assessment had to be submitted by 01.10.2023.
CAVE:
Please note that the specified deadlines for the risk assessment (steps 1, 2 and 3) as described above have expired. All marketing authorisation holders of human medicinal products with chemical active substances who have not yet reported identified impurities with nitrosamines should do so as a matter of priority, including any updates to previous notifications.
Biological active ingredients
Step 1 - Risk evaluation
If your biological proprietary medicinal product requires a risk evaluation regarding possible contamination with nitrosamines, the result of the risk evaluation must be submitted to the BASG by 1 July 2021 at the latest.
A corresponding procedure was created in the eService for each affected product. If this has not happened in individual cases, please contact The name of the medicinal speciality can be found in the subject line of each affected product (see Figure 1).
CMDh will provide you with 2 documents with the respective document designations (Step 1 - no risk identified, Step 1 - risk identified).
Please select the appropriate document for your product, fill it in completely and then upload it using the procedure provided in the eService, specifying the correct document type (Step 1 - no risk identified, Step 1 - risk identified) (see Figures 2 and 3).
To upload the documents, open the procedure via the respective procedure number and select voluntary subsequent submission.
The Excel table listed in the corresponding practical guidance (see CMDh website) is - as also stated there - not mandatory in Austria at this time and therefore does not necessarily have to be uploaded.
Please only use the procedure described above for your reports in Austria.
If you do not have access to the portal or if you have any questions, please contact
Step 2 - Testing
CAVE: only relevant if a risk was identified in Step 1.
In the eService, a corresponding procedure was created for each affected product with regard to Step 1 and - if a risk has been identified - this procedure remains open.
CMDh will provide you with 2 documents with the respective document designations (step 2 - no nitrosamines found, step 2 - nitrosamines found).
Please select the appropriate document for your product, fill it in completely and then upload it to the procedure provided in the eService, specifying the correct document type (step 2 - no nitrosamines found, step 2 - nitrosamines found) (see figure x and y).
To upload the documents, open the procedure via the respective procedure number and select voluntary subsequent submission.
Please use only the procedure described above for your notifications in Austria.
Step 3 - any changes concerning your medicinal product
Variations required due to the risk assessment had to be submitted by 01.07.2023.
CAVE:
Please note that the specified deadlines for the risk assessment (steps 1, 2 and 3) as described above have expired. All marketing authorisation holders of human medicinal products with biological active substances who have not yet reported identified impurities with nitrosamines should do so as a matter of priority, including any updates to previous notifications.
Enqueries:
E-mail: nat@basg.gv.at
Enqueries (for media): Communications Management, Tel.: 050555/25000
E-mail: presse-basg@basg.gv.at