Recommendations for preparation for Reg (EU) 536/2014
Training material from the European Medicines Agency
The European Medicines Agency (EMA) offers extensive training material to prepare for Regulation (EU) 536/2014 ("Clinical Trials Regulation, CTR") and for working in the Clinical Trials Information System (CTIS).
General information on the Clinical Trials Regulation (CTR)
Training material on the Clinical Trials Information System (CTIS)
Training materials include
- Online training modules
- Handbook for clinical trial sponsors
- Reference materials for clinical trial sponsors
- Reference materials for authorities
- Training and information events (including video recordings)
- Information on master trainers from sponsors, agencies and ethics committees
It is strongly recommended to be familiar with these training materials before submitting a clinical trial via CTIS.
User administration by the EMA
- In order to be able to take actions in the Clinical Trials Information System (CTIS) (e.g. submit an application), you have to be registered in the EMA's Account Management System. This is a self-registration which is open to all.
- Once you have an EMA account, you can request access to EMA applications such as CTIS, SPOR, IRIS, EudraVigilance and UPD on behalf of your organisation.
- If your organisation is not yet registered with EMA, you can do this yourself in EMA's Organisation Management Service (OMS). The prerequisite is that you are authorised to do so by your organisation, which will be checked by the EMA.
Important information for trial sites!
Trial sites also need to be registered as organisations in the EMA's OMS in order to be selected for a clinical trial application. This does not aim at the individual department, but at the organisational unit of the clinical institution.
A potential trial site therefore has to submit its Organisation ID (ORG ID) and the name and department of the Investigator to the Sponsor/Applicant prior to submitting an application in CTIS in order to be selected as a trial site. In CTIS first the organisation for the trial site is to be selected and then the Investigator's name, contact information and department are manually entered.
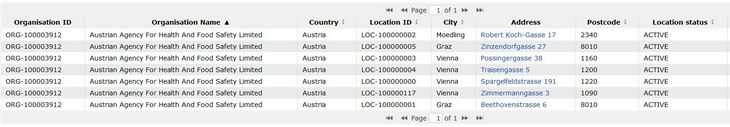
For complex organisational units it is recommended that the registration of the organisation is coordinated centrally (e.g. by the medical directorate or the rector). The administrator of the organization should be the person responsible for certifying the suitability of the clinical site as a trial site according to Annex I, letter N, of the regulation (although this responsibility can of course be delegated).
Using the example of the Agency for Health and Food Safety (AGES) below, one can see that the AGES Austrian Medicines and Medical Devices Agency (AGES MEA) located in Traisengasse, although it has its own site, is part of the overarching organisation AGES. In the case of a trial site, the AGES MEA would be the department and the AGES the clinic/institution.