Round table on "Availability of medicines in Austria" (2)
Measures to help secure the supply of medicines in Austria
Sales restrictions and supply difficulties, which can even lead to difficulties in the full supply of medicines, are increasing globally, throughout the EU and also in Austria.
On October 15, 2019 the "Round Table" on the availability of medicines in Austria took place.
More than 30 representatives of Austrian stakeholders in the health care system worked out solutions at the event, which was organised by the Federal Office for Safety in Health Care (BASG) on behalf of the BMASGK and attended by top-class participants. These solutions are intended to ensure that the supply situation in Austria can be maintained, safeguarded and strengthened in the future for the benefit of patients against the background of continuously increasing supply and supply bottlenecks worldwide.
Representatives from, among others, the Chamber of Physicians and Pharmacists, the Pharmaceutical Wholesale Trade, the Patients' Advocate's Office, the BMASGK, the Chamber of Commerce, the interested groups of the pharmaceutical industry as well as the scientific community and the Federation of Austrian Social Insurance Institutions were actively involved.
DI Dr. Christa Wirthumer-Hoche, procedural member of the BASG, chaired the event where concrete measures were developed which could soon be implemented.
Obligation to report medicine shortages
There was broad consensus that global production and supply shortages could only be solved to a limited extent at national level. It is therefore important to quickly bring transparency into the current supply situation and to communicate existing or imminent supply shortages to the occupational groups involved at an early stage.
In this way, countermeasures can be taken in good time in order to cushion the supply situation with individual measures as far as possible even in the event of long-term shortages and to set alternative supply steps in good time.
The reports on shortages, which have so far been handled only incompletely and voluntarily by marketing authorisation holders in many cases, should therefore be subject to a reporting obligation in order to improve the planning of the supply situation.
According to the plans, impending delivery difficulties that would last several weeks and would therefore no longer be bridgeable due to warehousing by wholesalers would have to be notified in advance to the Austrian Federal Office for Safety in Health Care (BASG) at the earliest possible stage.
In the event of impending shortages, it should also be possible to impose an export ban on the quantities of pharmaceuticals still available in Austria. In the past, there has been a tendency to sell medicines abroad, which in some cases deprived the Austrian population of access to them, and this measure may help to ensure the supply of the Austrian population.
The round table dealt with the following key points:
- Extension of the reporting obligation for supply shortages for marketing authorisation holders in the distribution restrictions register
- The marketing authorisation holder reports at CIP code level:
- Non-available medicinal products
- Medicinal products not sufficiently available
- In the shortages catalouge this information could be stored with a traffic light system.
- The data in the catalouge is reported and subsequently maintained by the marketing authorisation holder.
- Marketing authorisation holder reports if the medicinal product is not available for 2 weeks or not sufficiently available for 4 weeks
- Date of notification: immediately, from the time of knowledge of the actual marketing restriction
- Export ban on all medicines listed in the shortages catalouge for all stakeholders in the distribution chain (i.e. industry, wholesalers, pharmacies).
- Possibility for BASG - in agreement with the marketing authorisation holder - to include further products on the export ban list which are made available by the industry on an appropriate and continuous basis to cover the needs of patients in Austria, but which nevertheless cause supply problems (for other reasons such as unwanted discharge abroad by other stakeholders, etc.).
- Possibility of sanctions in the event of violations of export prohibitions, such as administrative penalties, GDP withdrawal, withdrawal of the license
- Electronic tools will be used to ensure that physicians do not prescribe medicines that are not available on a daily basis and that physicians and pharmacists are informed of medicinal products that are not available. This IT-technical solution will take some time.
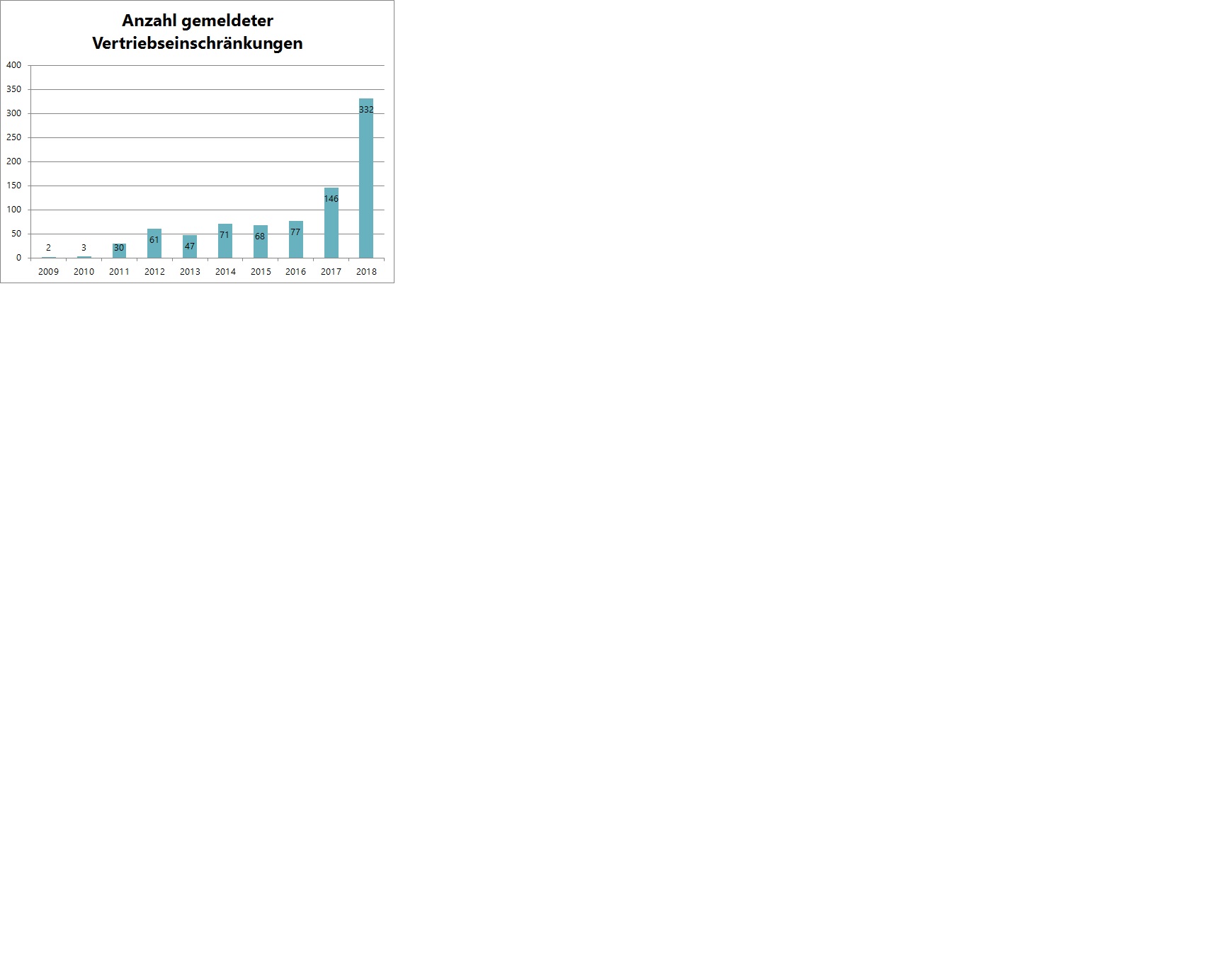
Problem Increase in sales restrictions in Austria:
Total number of medicine shortages (currently voluntary notifications): 2017: 146 and 2018: 332 (rising trend)
see graphic at end of article
Tasks and No-tasks of the BASG
The BASG is receiving more and more complaints about non-available medicines, but it must be made clear that the BASG cannot force pharmaceutical companies to produce medicines or active substances, nor can it oblige pharmaceutical companies to place medicines on the market.
Currently, the BASG can only investigate facts that have led to the restriction of distribution, communicate the extent and the information to the actors involved. In this context, reference will be made to the current tasks and non-tasks of the BASG which have been published:
However, solutions to the issues surrounding supply problems cannot be found at national level alone. Solutions must also be developed jointly in the EU, for which a joint HMA/EMA working group has been set up: https://www.hma.eu/522.html
In connection with distribution restrictions (medicine shortages) for medicinal products in Austria, the BASG provides information on its website in the Shortages Catalouge.
The primary responsibility for maintaining the supply capability of medicinal products lies with the marketing authorisation holder or wholesaler. It is based on the provision of § 57a. (1) Austrian Medicines Act:
"Der Zulassungsinhaber oder der Inhaber einer Registrierung einer Arzneispezialität und die Arzneimittel-Großhändler und Arzneimittel-Vollgroßhändler, die diese tatsächlich in Verkehr gebrachte Arzneispezialität vertreiben, haben im Rahmen ihrer jeweiligen Verantwortung eine angemessene und kontinuierliche Bereitstellung der Arzneispezialität für die Abgabe durch Apotheken oder für sonst zur Abgabe gemäß § 59 Berechtigte sicherzustellen, damit der Bedarf der Patienten im Inland gedeckt ist".
In principle, therefore, the primary responsibility for maintaining the supply capability of medicinal products lies with the marketing authorisation holder or wholesaler. It must be ensured that the needs of patients are covered domestically.
Unfortunately, in recent years there have been increasing restrictions on the sale of pharmaceuticals, not only in Austria, but also in the EU and worldwide. Since the risk of sales restrictions due to the progressing global development has not only theoretically increased in recent years, but is already actually increasing, suitable measures must be taken quickly to bring about a trend reversal.
Conclusion:
In the future, sales restrictions should be reported to the Federal Office for Safety in Health Care (BASG) in order to create more transparency, to identify shortages in good time and to be able to initiate countermeasures at an early stage.
An export ban in the case of particularly critical shortages in the interest of public health, with reference to the shortages catalouge, is envisaged to prevent the withdrawal from the Austrian health system of quotas for unwanted exports which are already threatened with shortages for Austria and which are still stored in Austria.
Further proposals for concrete measures have been discussed and are being dealt with in the Task Force set up on this subject.
Questions (for media):
Communication Management, Tel.: 050555/25000
E-mail: presse-basg@basg.gv.at
www.basg.gv.at/arzneimittel/vertriebseinschraenkungen-lieferengpaesse/