Warning about counterfeit medicinal product Ozempic®
The Federal Office for Safety in Health Care (BASG) informs about important current developments and necessary measures in case of counterfeiting of the drug Ozempic® of the original manufacturer Novo Nordisk in German presentation.
Ozempic® is a prescription medicinal product used to treat inadequately controlled type 2 diabetes mellitus in adults as an adjunct to diet and physical activity. It is known that Ozempic®, which contains the same active ingredient (semaglutide) as the prescription drug Wegovy® (which is approved as a "weight loss medicinal product"), is now increasingly being used off-label (i.e. prescription or dispensing/use not covered by the marketing authorisation) for weight loss. This has already led to significant national and international sales restrictions and partial supply problems for diabetics, for whom the drug is actually approved. Ozempic® has therefore already been listed by the BASG on both the medicine shortages catalogue and the parallel export ban catalogue.
Criminal organizations have apparently taken advantage of the situation and are trying to profit from this situation in a criminal and dangerous way by counterfeiting the high-priced product in a way that poses a risk to health. According to the current state of knowledge, counterfeits have been identified in packages of 1 mg strength, but it cannot be ruled out that other packages or active ingredient strengths are or will be affected.
So far, there are no findings that the counterfeits have reached patients in Austria. According to initial information, the counterfeits were probably intended for export outside the EU. However, it cannot be ruled out that such counterfeit medicinal product are also in the distribution chains in Austria.
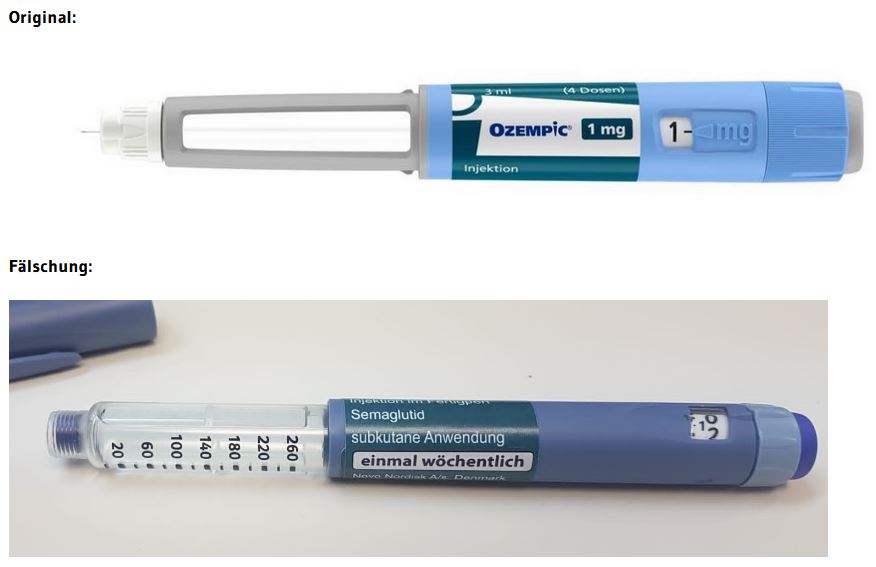
For the time being, the counterfeits identified so far can be easily distinguished from the originals on the primary packaging (=syringe) (see photo, original compared to the counterfeit. Dark blue color of the counterfeit syringe instead of light blue in the original, as well as blue syringe head of the counterfeit instead of gray in the original). On the basis of the secondary packaging (outer carton/packaging box), on the other hand, it is difficult or impossible to distinguish the counterfeits purely visually.
BASG advises all patients that Ozempic® is a prescription-only medicinal product. It is therefore not possible to order it on the internet, where only over-the-counter medicines can legally be obtained. Any order placed on the internet for Ozempic® is therefore not only unlawful and illegal, but carries a very high probability of obtaining a counterfeit Ozempic® product. These counterfeits can be hazardous to your health and potentially life-threatening due to their untested quality and potential contaminants and unknown ingredients. Legal mail order pharmacies (which by law are only ever authorized to ship over-the-counter medications) therefore do not ship Ozempic®.
Note: Legal mail-order pharmacies for ordering exclusively over-the-counter medicines can always be recognized by their officially registered and tested mail-order pharmacy logo - and thus distinguished from health-endangering "rogue pharmacies" - in contrast to the multitude of illegal pharmacies appearing on the Internet (so-called "rogue pharmacies"/illegal pharmacies that predominantly sell counterfeits).
Patients are therefore strongly and urgently warned against any unauthorized ordering of Ozempic® on the internet. Genuine Ozempic® can only be obtained by prescription and dispensing through a public pharmacy. This is the only way to ensure that the product purchased is an approved, well-tested, safe and effective and therefore ultimately authentic medicinal product.
BASG advises all pharmacists that before dispensing the medicinal product to patients, the secondary packaging must always be checked for authenticity using the electronic security feature as part of the serialization and verification of medicinal products. Any suspicion that a medicinal product is counterfeit can and should be reported immediately to the BASG. As in the case of Ozempic®, the BASG carefully investigates all facts and suspicions concerning counterfeit medicines.
The present case is currently already the subject of official investigations and the BASG is cooperating closely with the other investigating authorities involved at national and international level.
Enquiries (professional):
Dr. Christoph Baumgärtel, Tel.: +43 505 55-36004
E-Mail: christoph.baumgaertel@ages.at
Enquiries (for media):
Communication Management, Tel.: +43 505 55-25000
E-Mail: presse-basg@basg.gv.at
| Further information Link | BASG warnings, updates 19/10/2023 and 23/10/2023 |
|---|