Quality of medicines
Assessment of Certificates of Suitability (CEP)
BASG is involved in the assessment of CEPs („Certificate of Suitability of the European Pharmacopoeia“) as one of the most active national competent authorities.
The application dossier for a medicinal product has to provide data on the quality, safety and efficacy of the medicinal product. In terms of quality, extensive data on both the active substance and the finished product have to be provided.
To avoid multiple assessments of identical active substance documentations by different authorities during different procedures, the documentation on an active substance can be submitted to the European Directorate for the Quality of Medicines & HealthCare (EDQM). After a positive centralised assessment by assessors from national competent authorities and EDQM, a CEP is granted; the CEP certifies that the quality of the active substance in question is adequately documented. Future application dossiers only need to include a copy of the CEP rather than an extensive active substance documentation, eliminating the need for repeat assessments of the active substance.
Furthermore, the same procedure is in place to evaluate TSE (Transmissible Spongiform Encephalopathy) risk of active substances and excipients and hereby helps to guarantee TSE-free products.
Currently, Austria is sending 10 experienced assessors to the CEP working party of EDQM (nine assessors regarding chemical evaluations and one assessor regarding TSE evaluation). Since years Austria is among the most active national competent authorities within Europe. In 2013 for the very first time the top position was reached meaning that no other country sent more assessors to Strasbourg than Austria did. EDQM not only covers travel and accommodation expenses, furthermore, the loss of assessing capacities is reimbursed to the national competent authorities.
In addition, Austria is represented in the Technical Advisory Board and, as a result, can actively participate in and shape professional and strategic decisions. Furthermore, an Austrian delegate is also member of the “Ad Hoc Committee” and thereby involved in discussions and decisions as regards CEP suspensions and withdrawals.
As of 2020, the EDQM revised the CEP to improve the usability and transparency of the CEP. The resulting CEP 2.0 was implemented on September 1, 2023.
Further Information
Database for information on CEPs granted by the EDQM: Search Certification Database
Information regarding suspended and withdrawn CEPs: Actions on CEPs
Information regarding CEP 2.0: CEP 2.0
Validity of the Active Substance Manufacturer and Finished Product Manufacturer
The Austrian Federal Office for Safety in Health Care (BASG) points out that marketing authorisation holders are obliged to ensure that the quality of their medicinal products is in accordance with Article 4 of the Austrian Medicines Act (Arzneimittelgesetz, AMG).
Active Substance
In cases where the quality of an active substance is documented by an EDQM certificate (CEP), the continuous validity of the certificate has to be ensured. Certificates transiently suspended or declared invalid as a result of negative GMP inspections are listed on the EDQM website (Actions on CEPs) or can be queried from the Certification database.
Analogously, the quality of an active substance referring to an Active Substance Master File (ASMF) cannot be ensured after GMP inspections with negative outcome. Corresponding Non-Compliance Reports (NCRs) are published under http://eudragmdp.ema.europa.eu/.
In both cases, the quality of the active substances concerned can, from the viewpoint of BASG, no longer be ensured and, as a result, fails to meet the requirements of the Austrian Medicines Act.
If necessary, a different/new active substance manufacturer including respective documentation has to be approved via variation procedure.
Finished Product
If a GMP-inspection of a finished product manufacturer has a negative outcome, a corresponding Non-Compliance Report (NCR) will be published under http://eudragmdp.ema.europa.eu/. The quality of medicinal product(s) concerned can, from the viewpoint of BASG, no longer be ensured and, as a result, fails to meet the requirements of the Austrian Medicines Act.
If necessary, a different/new finished product manufacturer including respective documentation has to be approved via variation procedure.
In case of questions regarding NCRs please contact am-qualitaetsmangel@basg.gv.at, in case of questions regarding concerned products please contact lcm@basg.gv.at.
Risk assessment of nitrosamines in medicinal products
Information for marketing authorisation holders: Request to evaluate the risk of the presence of nitrosamine impurities in human medicinal products.
Nitrosamines are chemical compounds classified as probable human carcinogens on the basis of animal studies. After nitrosamine impurities have been found in various medicinal products a scientific evaluation by the Committee for Medicinal Products for Human Use (CHMP) was initiated in September 2019 in accordance with Article 5(3) of Regulation (EC) No 726/2004, which was finalised in July 2020.
As a result, it has been established that the risk of the presence of nitrosamines as impurities in human medicinal products must be reduced as much as possible and must be at or below the applicable limit.
Further on, all marketing authorisation holders of human medicinal products containing chemically synthesized or biological active substances are required to review their products regarding the risk of presence of nitrosamine impurities and to take measures if necessary.
Further information can be found
- on the homepage of the European Medicines Agency: EMA - Nitrosamine Impurities
- and the Coordination Group: CMD(h) - Nitrosamine Impurities
Practical Guidance Austria in relation to the Art. 5(3) Referral on Nitrosamines
The risk assessment should be performed in 3 sequential steps
- Step 1 – risk evaluation
- Step 2 – if applicable, confirmatory testing
- Step 3 – if applicable, changes to the marketing authorisation
Human Medicinal Products
Chemically Syntetised Active Substances
Step 1 – risk evaluation
Results of the risk evaluation in regard to nitrosamine impurities had to be submitted until 31 March 2021 to BASG for all authorised human medicinal products containing chemically synthesised active pharmaceutical ingredients.
A procedure is provided in eServices for all products concerned. If this has not happened in your case please contact nat@basg.gv.at.
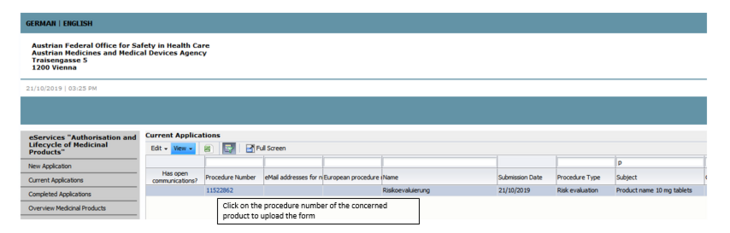
The procedure is to be found by name Risikoevaluierung, the name of the medicinal product is to be found by subject in connection to each product concerned (see picture 1).
You will be provided with 2 documents by CMDh with the respective document names (step 1 - no risk identified, step 1 - risk identified).
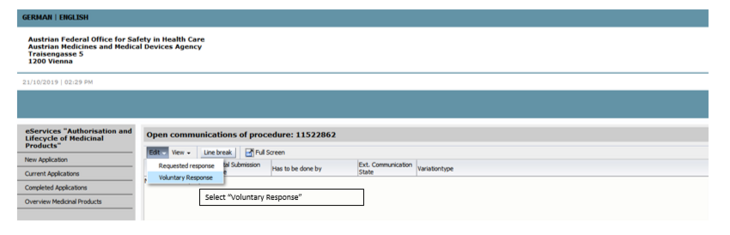
Please choose the document suitable for your marketing authorisation, fill in this document completely and upload it to eService (see picture 2 and 3) by using the correct type of document (step 1 – no risk identified, step 1 – risk identified). To upload the document please open the procedure and choose voluntary response.
The Excel sheet mentioned in the practical guide (see CMDh website) is currently not necessary for Austria and has therefore not to be uploaded to eServices.
Please use for submission of the results of the risk evaluation for Austria only the procedure described above.
If you do not have access to the Austrian eServices or in case of questions please contact nat@basg.gv.at.
Step 2 – Testing
CAVE: only relevant if a risk has been identified in Step 1.
A procedure is provided in eServices for all products concerned and –if a risk has been identified- the procedures stays open. If this has not happened in your case please contact nat@basg.gv.at. The procedure is to be found by name Risikoevaluierung, the name of the medicinal product is to be found by subject in connection to each product concerned (see picture 1).
You will be provided with 2 documents by CMDh with the respective document names (step 1 - no risk identified, step 1 - risk identified).
Please choose the document suitable for your marketing authorisation, fill in this document completely and upload it to eService (see picture 2 and 3) by using the correct type of document (step 2 - no nitrosamine detected, step 2 - nitrosamine detected).
To upload the document please open the procedure and choose voluntary response.
Please use for submission of the results of the risk evaluation for Austria only the procedure described above.
Step 3 – changes to the medicinal product
Changes necessary due to performed the risk assessment had to be submitted by October 1st, 2023.
CAVE:
Please note that set deadlines for risk assessment (Step 1, 2 and 3) as described above for medicines containing chemically synthesised active substances have passed. Any marketing authorisation holders that have not yet reported identified nitrosamine impurities, should do so as a matter of priority, including any updates to previous notifications.
Biologicals Step 1 – risk evaluation
If your biological medicinal product requires a risk evaluation regarding possible nitrosamine contamination, the result of the risk evaluation had to be submitted to the BASG by 01.07.2021.
A procedure is provided in eServices for all products concerned. If this has not happened in your case please contact nat@basg.gv.at.
The procedure is to be found by name Risikoevaluierung, the name of the medicinal product is to be found by subject in connection to each product concerned (see picture 1).
CMDh will provide you with 2 documents with the respective document designations (step 1 - no risk identified, step 1 - risk identified).
Please choose the document suitable for your marketing authorisation, fill in this document completely and upload it to eService (see picture 2 and 3) by using the correct type of document (step 1 – no risk identified, step 1 – risk identified).
To upload the document please open the procedure and choose voluntary response.
The Excel spreadsheet mentioned in the corresponding practical guidance (see CMDh website) is - as also indicated there - not mandatory required in Austria at the moment and therefore not necessarily to be uploaded.
Please use only the procedure described above for your reports in Austria.
If you do not have access to the portal or if you have any questions, please contact nat@basg.gv.at.
Step 2 – Testing
CAVE: only relevant if a risk has been identified in Step 1.
A procedure is provided in eServices for all products concerned and –if a risk has been identified- the procedures stays open. If this has not happened in your case please contact nat@basg.gv.at. The procedure is to be found by name Risikoevaluierung, the name of the medicinal product is to be found by subject in connection to each product concerned (see picture 1).
You will be provided with 2 documents by CMDh with the respective document names (step 1 - no risk identified, step 1 - risk identified).
Please choose the document suitable for your marketing authorisation, fill in this document completely and upload it to eService (see picture 2 and 3) by using the correct type of document (step 2 – no nitrosamine detected, step 2 – nitrosamine detected ).
To upload the document please open the procedure and choose voluntary response.
Please use for submission of the results of the risk evaluation for Austria only the procedure described above.
Step 3 – changes to the medicinal product
Changes necessary due to the performed risk assessment had to be submitted by July 1st, 2023.
CAVE:
Please note that set deadlines for risk assessment (Step 1, 2 and 3) as described above for medicines containing biological active substances have passed. Any marketing authorisation holders that have not yet reported identified nitrosamine impurities, should do so as a matter of priority, including any updates to previous notifications.
Enqueries:
E-mail: nat@basg.gv.at
Enqueries (for media): Communications Management, Tel.: 050555/25000
E-mail: presse-basg@basg.gv.at